
CYSTARAN® Clinical Studies

CYSTARAN® Clinical Studies

CYSTARAN® Clinical Studies
Study 1

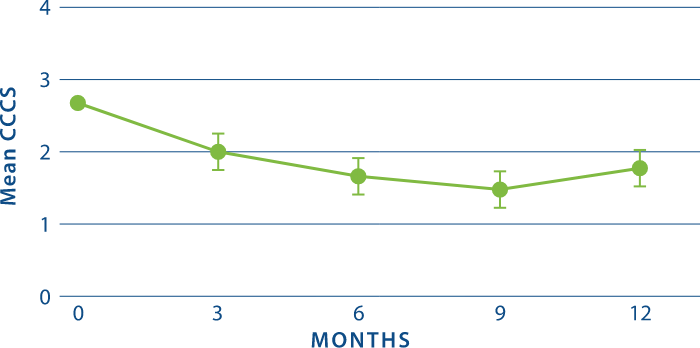
*STUDY DESIGN: Multicenter, randomized, double-blind efficacy trial of CYSTARAN in 15 treatment-naïve patients with a baseline CCCS of ≥ 1.25. The primary end point was the estimated proportion of eyes with a CCCS reduction ≥1 relative to baseline (where baseline CCCS was ≥1) anytime during the treatment period and at Months 3, 6, 9, and 12. Slit-lamp photography was used to assess CCCS changes from baseline.1,2
†CCCS = Corneal cystine crystal score, a measure of crystal density assessed using slit-lamp photography. CCCS ranges from 0 units (clear at the center) to 3 units (highest crystal density).
Rapid CCCS Reductions as Early as 3 Months and Sustained Through 1 Year2

Study 2
In the combined analysis of 3 clinical studies‡, patients treated with CYSTARAN showed
Study 2
In the combined analysis of 3 clinical studies‡, patients treated with CYSTARAN showed
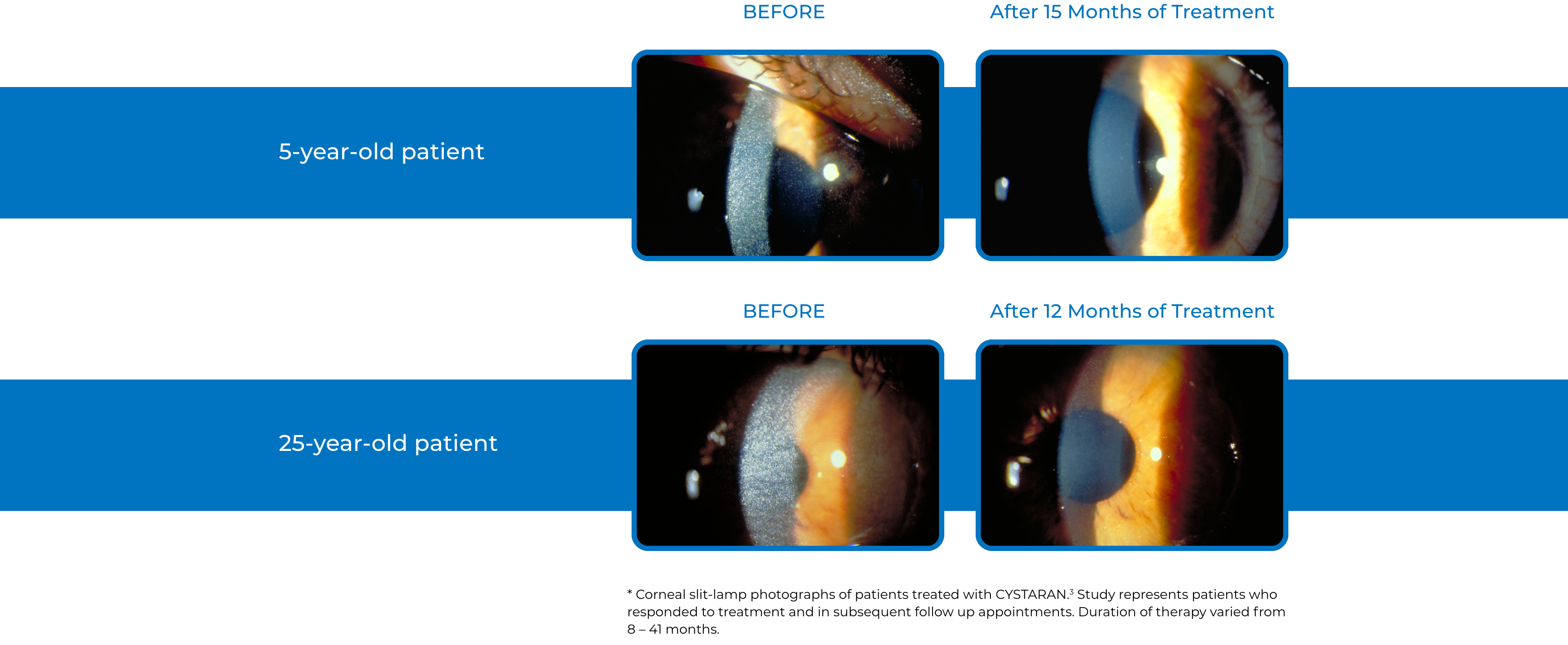
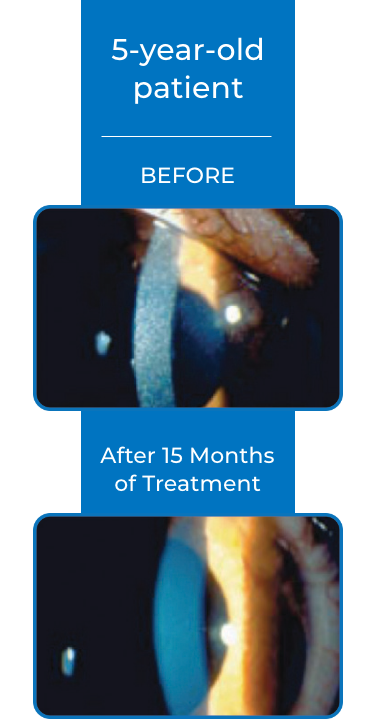
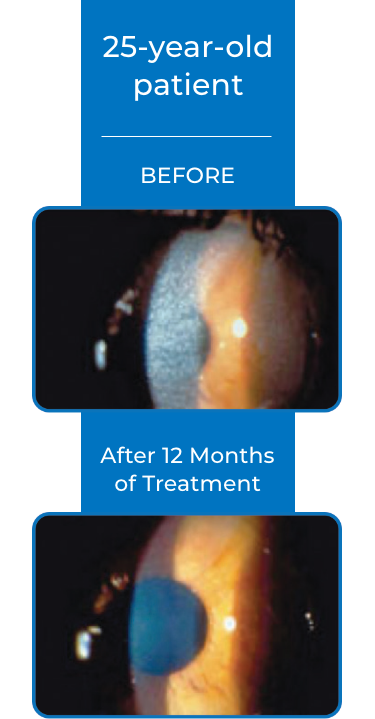
‡STUDY DESIGN: In the Combined Analysis of Patients Treated with Ophthalmic Cysteamine (CAPTOC) study, 247 patients were enrolled. Of these, 161 patients were the mITT population (defined as patients with CCCS values at baseline and post baseline timepoints). The primary end point was reduction of CCCS in eyes with high (≥1) CCCS at baseline and lack of increase in CCCS in eyes with low (<1) CCCS at baseline. End points were based on photo-rated CCCS (slit-lamp photography in conjunction with a photography-based scoring system) to quantify and document corneal cystine crystal accumulation over time.2
Examples of Treatment with CYSTARAN3*
Safety Data1
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data described below reflect exposure in controlled clinical trials of six months to 19 years duration in approximately 250 patients.
Most frequently reported ocular adverse reactions (occurring in ≥10% of patients)
- Sensitivity to light
- Redness
- Eye pain/irritation
- Headache
- Visual field defects
Safety Data1
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data described below reflect exposure in controlled clinical trials of six months to 19 years duration in approximately 250 patients.
Most frequently reported ocular adverse reactions (occurring in ≥10% of patients)
- Sensitivity to light
- Redness
- Eye pain/irritation
- Headache
- Visual field defects


References: 1. CYSTARAN [prescribing information]. Gaithersburg, MD: Leadiant Biosciences, Inc.; Current approved PI. 2. Data on File. Leadiant Biosciences, Inc. 3. Gahl WA, Kuehl EM, Iwata F, Lindblad A, Kaiser-Kupfer MI. Corneal Crystals in Nephropathic Cystinosis: Natural History and Treatment with Cysteamine Eyedrops. Molec Genet Metab. 2000;71:100-120
INDICATION & IMPORTANT SAFETY INFORMATION
CYSTARAN® (CYSTEAMINE OPHTHALMIC SOLUTION) 0.44% is a cystine-depleting agent indicated for the treatment of corneal cystine crystal accumulation in patients with cystinosis.
IMPORTANT SAFETY INFORMATION
- To minimize contaminating the dropper tip and solution, care should be taken not to touch the eyelids or surrounding areas with the dropper tip of the bottle. Keep bottle tightly closed when not in use.
- There have been reports of benign intracranial hypertension (or pseudotumor cerebri) associated with oral cysteamine treatment that has resolved with the addition of diuretic therapy. There have also been reports associated with ophthalmic use of cysteamine; however, all of these patients were on concurrent oral cysteamine.
- CYSTARAN contains benzalkonium chloride, which may be absorbed by soft contact lenses. Contact lenses should be removed prior to application of solution and may be reinserted 15 minutes following its administration.
- CYSTARAN is for topical ophthalmic use only.
- The most frequently reported ocular adverse reactions occurring in ≥ 10% of patients were sensitivity to light, redness, and eye pain/irritation, headache and visual field defects.
Please refer to CYSTARAN’s full Prescribing Information, available here.




