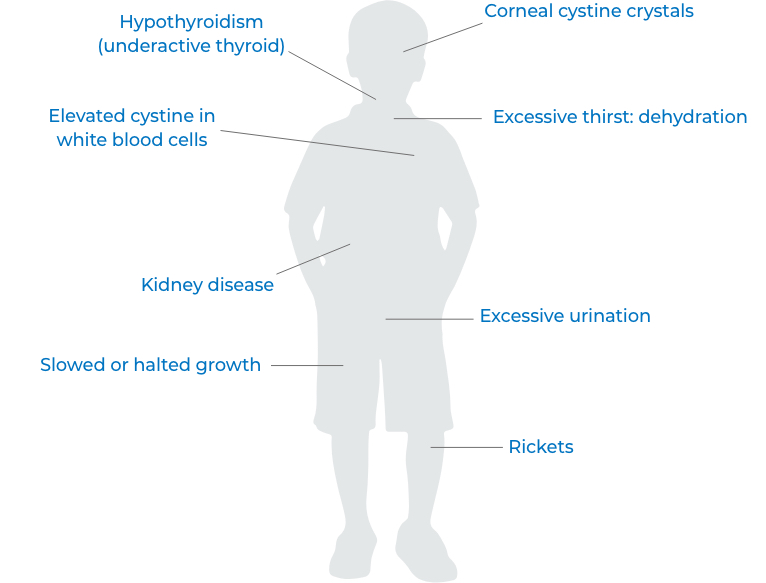
What is CYSTARAN®?

What is CYSTARAN®?

What is
CYSTARAN®?
What is CYSTARAN?
CYSTARAN is an eyedrop medication intended to treat the buildup of cystine crystals in the corneas of adults and children who have cystinosis. It was approved by the FDA in 2012 as an orphan drug.

CYSTARAN is a topical medication, which means that you put it directly on the affected area—in this case, the eyes. That’s different from an oral medication, which you take by mouth. If you have cystinosis, you may be prescribed both an oral and a topical medication.
How does CYSTARAN work?
Cysteamine, the active ingredient in CYSTARAN, works to help decrease the accumulation of cystine crystals in the body’s tissues.
Cysteamine binds to and changes cystine. The bound substance can be removed from the lysosomes of the body’s cells without a need for cystinosin and is not harmful to the eye.
Lysosome Affected by Cystinosis
Lysosome Affected by Cystinosis
After Taking Cysteamine


The use of CYSTARAN has been demonstrated to be effective at reducing corneal crystals.
How does CYSTARAN work?
Cysteamine, the active ingredient in CYSTARAN, works to help decrease the accumulation of cystine crystals in the body’s tissues.
Cysteamine binds to and changes cystine. The bound substance can be removed from the lysosomes of the body’s cells without a need for cystinosin and is not harmful to the eye.
Lysosome Affected by Cystinosis

Lysosome Affected by Cystinosis After Taking Cysteamine

The use of CYSTARAN has been demonstrated to be effective at reducing corneal crystals.
See examples of Treatment with CYSTARAN*
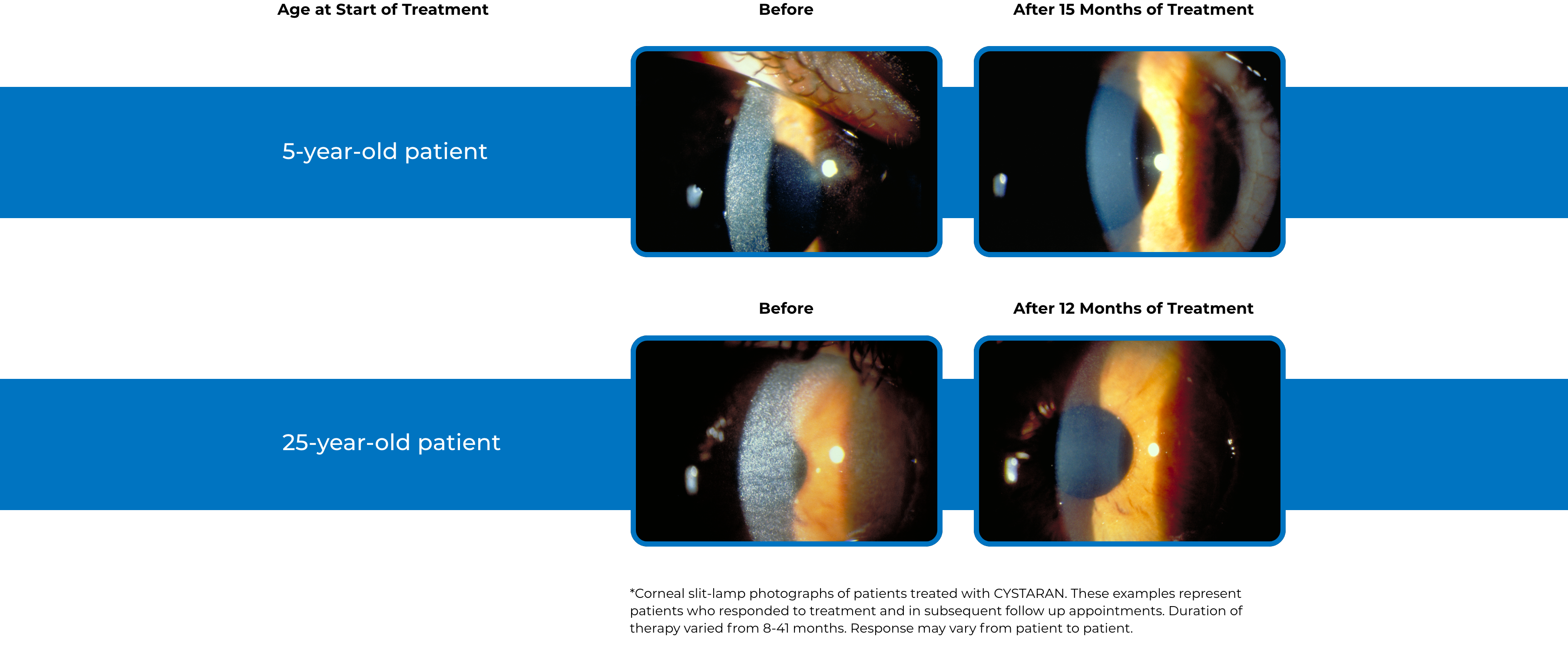
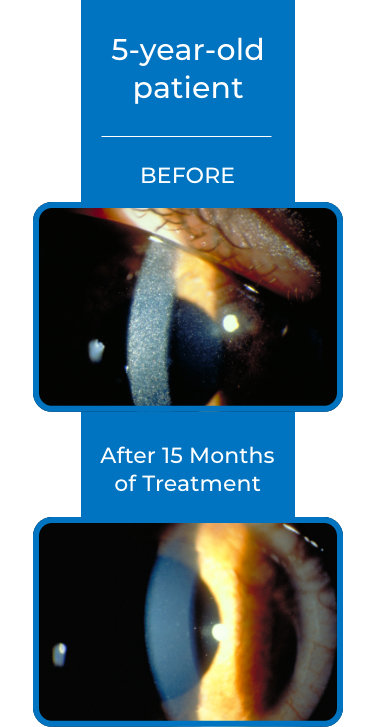
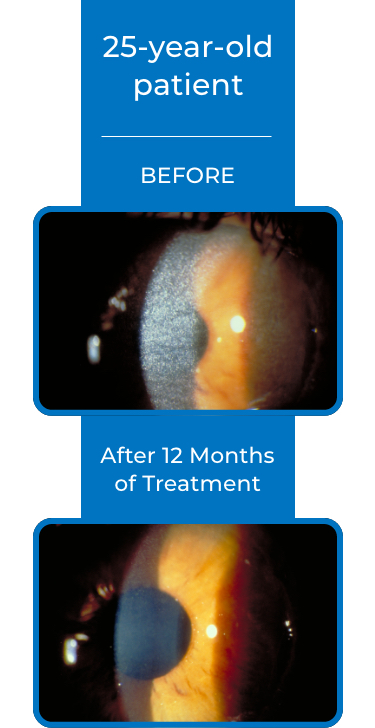
*Corneal slit-lamp photographs of patients treated with CYSTARAN. These examples represent patients who responded to treatment and in subsequent follow up appointments. Duration of therapy varied from 8-41 months. Response may vary from patient to patient.
How do I store and take CYSTARAN?
How to Store CYSTARAN
Store bottles in the freezer in the original carton.
Each week, remove one new bottle from the freezer.
Allow the bottle to thaw completely (approximately 24 hours) prior to use.
After the bottle is completely thawed, record the discard date on the bottle label. The discard date is seven (7) days from the day the bottle is thawed.
Store the thawed bottle at no greater than room temperature for up to 1 week. No refrigeration required after thawing. Do not refreeze the bottle.
Remember to discard the bottle at the end of 1 week (7 days). There may be medication left in the bottle; however, you must discard it because the medication is only stable for 1 week after thawing.
Store bottles in the freezer in the original carton.
Each week, remove one new bottle from the freezer.
Allow the bottle to thaw completely (approximately 24 hours) prior to use.
After the bottle is completely thawed, record the discard date on the bottle label. The discard date is seven (7) days from the day the bottle is thawed.
Store the thawed bottle at no greater than room temperature for up to 1 week. No refrigeration required after thawing. Do not refreeze the bottle.
Remember to discard the bottle at the end of 1 week (7 days). There may be medication left in the bottle; however, you must discard it because the medication is only stable for 1 week after thawing.

How to Take CYSTARAN

Instill one drop of CYSTARAN in each eye, every waking hour
Do not touch dropper tip to any surface, as this may contaminate the solution
Discard after
1 week of use
There may be medication left in the bottle; however, the bottle must be discarded by the patient because the medication is only stable for 1 week after thawing
How to Take CYSTARAN

Instill one drop of CYSTARAN in each eye, every waking hour
Do not touch dropper tip to any surface, as this may contaminate the solution
Discard after 1 week of use
There may be medication left in the bottle; however, the bottle must be discarded by the patient because the medication is only stable for 1 week after thawing

Where do I order CYSTARAN?
CYSTARAN is ONLY available from AllianceRx Walgreens Prime Pharmacy.
Your medication will be shipped directly to your home.
To order, call 1-877-534-9627
Friendly associates exclusively available to help CYSTARAN patients will be happy to assist you Mon–Fri, 8AM–7PM EST.
To order, call 1-877-534-9627
Friendly associates exclusively available to help CYSTARAN patients will be happy to assist you Mon–Fri, 8AM–7PM EST.
Patient Support Services
Leadiant Biosciences, Inc. is committed to helping patients and families access our products, including CYSTARAN. Learn what advocacy and assistance services are available to cystinosis patients here.
Frequently Asked Questions (FAQs)
IMPORTANT SAFETY INFORMATION
What is CYSTARAN?
CYSTARAN (cysteamine ophthalmic solution) 0.44% is an eyedrop medication used to treat cystine crystal accumulation in the corneas of patients who have cystinosis.
What is the most important safety information I should know about CYSTARAN?
- To help prevent contamination of the dropper tip and eyedrop medication, try to make sure that CYSTARAN is dropped directly onto the eye without touching it. Try not to touch the eyelids or surrounding areas with the dropper tip of the bottle when you are using CYSTARAN. Keep the bottle tightly closed when not in use.
- CYSTARAN contains an ingredient called benzalkonium chloride which can be absorbed by soft contact lenses. Remove contact lenses before using CYSTARAN eyedrops and wait at least 15 minutes before reinserting them.
- CYSTARAN should only be used as an eyedrop medication.
What are the side effects of CYSTARAN?
- The most common side effects of CYSTARAN, which have occurred in at least 10% of people using the medication, were sensitivity to light, eye redness, eye pain and irritation, and headache.
The risk information provided here is not comprehensive. To learn more, talk to your healthcare provider or pharmacist about CYSTARAN. The full FDA-approved product labeling can be found at www.cystaran.com.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call FDA at 1-800-FDA-1088.