
What is Cystinosis?

What is Cystinosis?

What is
Cystinosis?
What is cystinosis?
Cystinosis is a rare but serious multi-system genetic disorder that initially manifests in the kidneys. If cystinosis goes untreated, it can be fatal.
Cystinosis is now considered manageable with treatment thanks to the availability of effective medication.

How CTNS Defects Cause Cystinosis
Cystinosis is caused by a genetic defect in the CTNS gene, which tells the body how to build an essential transporter protein called cystinosin. This defect is usually a deletion of a specific portion of the gene—the 57-kb segment. However, other types of defects on the CTNS gene or varied levels of deletion exist. These variations are responsible for how the disease can behave differently in different people.
Functioning CTNS Gene
Defective CTNS Gene

Defects in the CTNS gene prevent cystinosin from working properly or from being produced in the right amounts.
How CTNS Defects Cause Cystinosis
Cystinosis is caused by a genetic defect in the CTNS gene, which tells the body how to build an essential transporter protein called cystinosin. This defect is usually a deletion of a specific portion of the gene—the 57-kb segment. However, other types of defects on the CTNS gene or varied levels of deletion exist. These variations are responsible for how the disease can behave differently in different people.
Functioning CTNS Gene

Defective CTNS Gene

Defects in the CTNS gene prevent cystinosin from working properly or from being produced in the right amounts.
When cystinosin is not able to transport cystine, cystine begins to build up in cells, eventually forming crystals throughout the body:
Normal Lysosome
In a normal cell, cystinosin helps remove cystine from the cell’s lysosome, the part of the cell that digests and recycles materials

Mutated Lysosome
When cystinosin doesn’t work properly or isn’t present in the right amounts, cystine builds up in the lysosomes throughout the body—including in the eyes

Lysosomes are found inside of cells and contain enzymes which break down molecules into smaller pieces so that they can be recycled for other uses or carried away.
When cystinosin is not able to transport cystine, cystine begins to build up in cells, eventually forming crystals throughout the body:
Normal Lysosome
In a normal cell, cystinosin helps remove cystine from the cell’s lysosome, the part of the cell that digests and recycles materials

Mutated Lysosome
When cystinosin doesn’t work properly or isn’t present in the right amounts, cystine builds up in the lysosomes throughout the body—including in the eyes

Lysosomes are found inside of cells and contain enzymes which break down molecules into smaller pieces so that they can be recycled for other uses or carried away.
Recognized Types of Cystinosis
There are three types of cystinosis that have been recognized by doctors:
Nephropathic cystinosis
Other terms: Infantile cystinosis, classic cystinosis
- 95% of reported cases are this type; most severe
- Appears in infancy
- Affects the kidneys and other organ systems
Intermediate cystinosis
Other terms: Juvenile cystinosis, late-onset cystinosis
- Unknown how many cases are of this type
- Appears later in life
- Also affects the kidneys and other organ systems but may progress more slowly in the body than the most severe type of cystinosis
Non-nephropathic cystinosis
Other terms: Ocular cystinosis, benign cystinosis, adult cystinosis
- Unknown how many cases are of this type
- Only the corneas of the eyes have crystal accumulation; kidneys and other organs are not affected


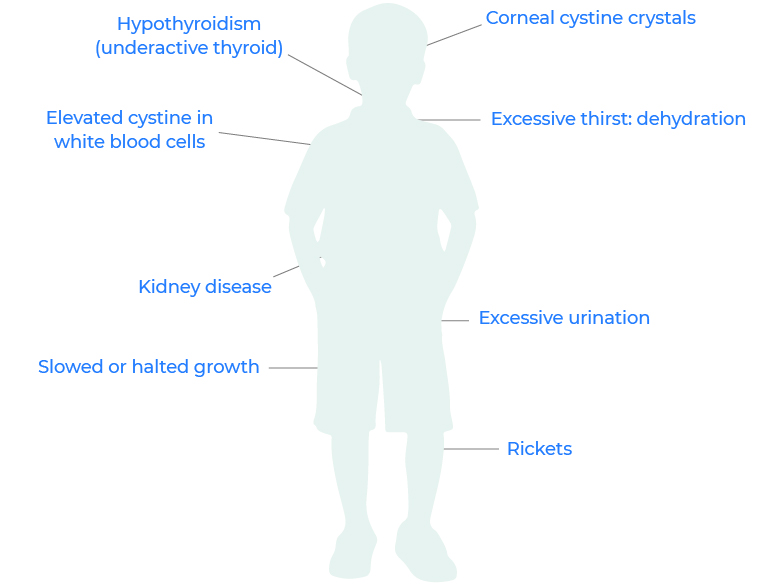
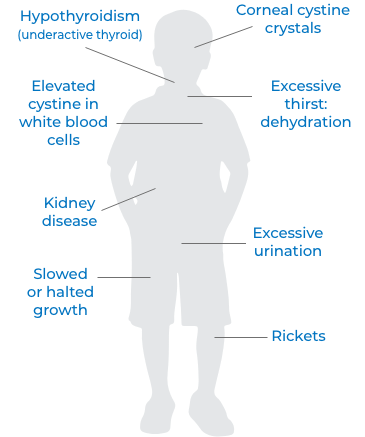
What are the early signs and symptoms of cystinosis?
Cystinosis can affect many different parts of the body. The most common and severe type causes a form of kidney disease (Fanconi’s syndrome) during infancy.
Early signs and symptoms of cystinosis include:
How does cystinosis affect eye health?
The underlying genetic problem that causes cystinosis is known to affect the eyes. In fact, cystinosis is a potentially sight-threatening condition due to disease affecting the retina.
How Cystinosis Affects the Eye
Anterior (Front 1/3 of Eye)
- When corneal crystals are left to accumulate, they can lead to potentially vision-impairing corneal scarring
- Buildup can also lead to chronic red eye, corneal scrapes or injury, inflammation, and even glaucoma
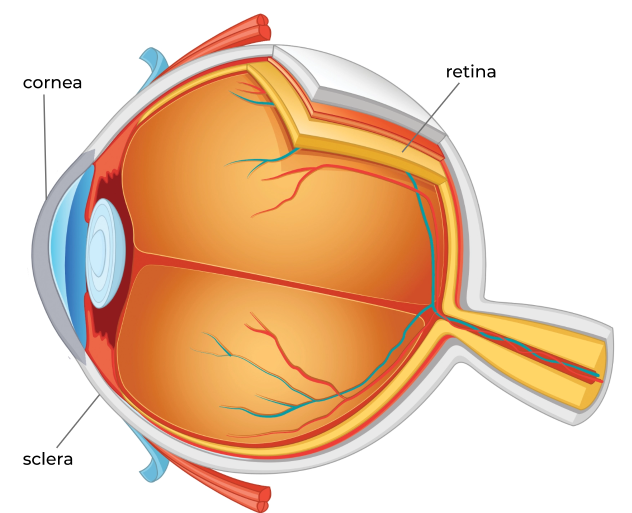
Posterior (Back 2/3 of Eye)
- Cystinosis can cause a decrease in the quality and field of vision, night vision, and even perception of colors because of accumulation in the retina
- If the retina is damaged enough, significant visual impairment is possible
What symptoms should I watch out for?
There are several symptoms that may be signs of cystine crystal accumulation in your corneas.
Leaving corneal crystal accumulation untreated may put your eyes at risk for serious issues later on. If you’re experiencing these symptoms, talk to your ophthalmologist immediately:
- Sensitivity to light (this is typically the first and most common symptom of crystal accumulation)
- Constant squinting and muscle spasms in the eyelids
- Persistent red eye
- Feeling like something is “in your eye”
- Eye pain
What symptoms should I watch out for?
There are several symptoms that may be signs of cystine crystal accumulation in your corneas.
![]() Leaving corneal crystal accumulation untreated may put your eyes at risk for serious issues later on.
Leaving corneal crystal accumulation untreated may put your eyes at risk for serious issues later on.
If you’re experiencing these symptoms, talk to your ophthalmologist immediately:
- Sensitivity to light (this is typically the first and most common symptom of crystal accumulation)
- Constant squinting and muscle spasms in the eyelids
- Persistent red eye
- Feeling like something is “in your eye”
- Eye pain


Why should eyes be treated?
If you are already taking oral medication for infantile or intermediate nephropathic cystinosis, that’s great! While oral cystinosis medication may help treat crystals in some parts of the eye, it can’t reach the cornea because there is no blood supply to the cornea to deliver the drug.
That means keeping eyes healthy with a cystinosis diagnosis can’t just be a matter of taking your oral medication as directed. To fight corneal crystal accumulation, you also need a therapy that goes directly on the eye, such as an eyedrop.
IMPORTANT SAFETY INFORMATION
What is CYSTARAN?
CYSTARAN (cysteamine ophthalmic solution) 0.44% is an eyedrop medication used to treat cystine crystal accumulation in the corneas of patients who have cystinosis.
What is the most important safety information I should know about CYSTARAN?
- To help prevent contamination of the dropper tip and eyedrop medication, try to make sure that CYSTARAN is dropped directly onto the eye without touching it. Try not to touch the eyelids or surrounding areas with the dropper tip of the bottle when you are using CYSTARAN. Keep the bottle tightly closed when not in use.
- CYSTARAN contains an ingredient called benzalkonium chloride which can be absorbed by soft contact lenses. Remove contact lenses before using CYSTARAN eyedrops and wait at least 15 minutes before reinserting them.
- CYSTARAN should only be used as an eyedrop medication.
What are the side effects of CYSTARAN?
- The most common side effects of CYSTARAN, which have occurred in at least 10% of people using the medication, were sensitivity to light, eye redness, eye pain and irritation, and headache.
The risk information provided here is not comprehensive. To learn more, talk to your healthcare provider or pharmacist about CYSTARAN. The full FDA-approved product labeling can be found at www.cystaran.com.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call FDA at 1-800-FDA-1088.



